elements and compounds are two types of
An element is any material made up of one type of atom- there could be multiples of this atom though for. Elements and compounds are two types of _____.
 |
| Compound Vs Mixture Difference And Comparison Diffen |
A compound is a pure substance made up of two or more kinds of elements that are chemically combined.

. Covalent or molecular compounds form when elements share electrons in a covalent bond to form molecules. Elements Compounds and Types of Mixtures. The atoms in compounds join together by chemical bonds. Covalent bonds and ionic bonds.
In the case of honey while there might be compounds in the honey like the various. In salts it is held together with ionic bonds. Covalent or molecular compounds form when elements share electrons in a covalent bond to form molecules. Step 1 1 of 2.
Compounds are made up of two or more elements. Pure substances which include elements and compounds have definite properties such as appearance texture. 1 See answer Advertisement Advertisement. The molecular formula of a covalent.
Also these are pure substances that exist in nature. Ionic compounds are usually. Chemical bonds link elements together to form more. These can be covalent bonds or ionic bonds.
Compounds consist of different elements bonded by a chemical reaction in a fixed ratio. No honey is not a compound. Compounds are the combinations of two or more elements with strong or weak attractive forces between them. When they combine the atoms of one element make.
Chemical compounds contain two or more types of atoms elements. An ionic compound that contains only two elements one present as a cation and one as an anion is called a binary ionic compound. Each atom type contains the same number of protons. Elements and compounds are made up of only one type of atom and two or more elements and compounds respectively.
In molecular compounds the atom binds each other through covalent bonds. They cannot be broken down into simpler fragments and can exist in. 10 Differences Between Compounds and Elements 1. Terms in this set 10 What is an elements.
Molecular compounds are electrically neutral. Elements are pure substances that cannot be broken down into. Example Of Compounds See more. Iron Silver Copper are some examples of.
Elements and compounds are two types of _____. A compound is when two or more substances bond chemically. Compounds can be classified into two types molecular compounds and salts. There are 2 types of bonds.
These are the two types of bonds out of which every compound is made of. Elements and compounds are two types of substances I think. A pure substance composed of atoms of two or more elements bonded chemically with one another in a definite proportion is known as a compound. An element is a material that consists of a single type of atom.
The difference between elements and compounds is part of a very important understanding in the study of chemistry. They have only one type of molecule. Molecular compounds are electrically neutral.
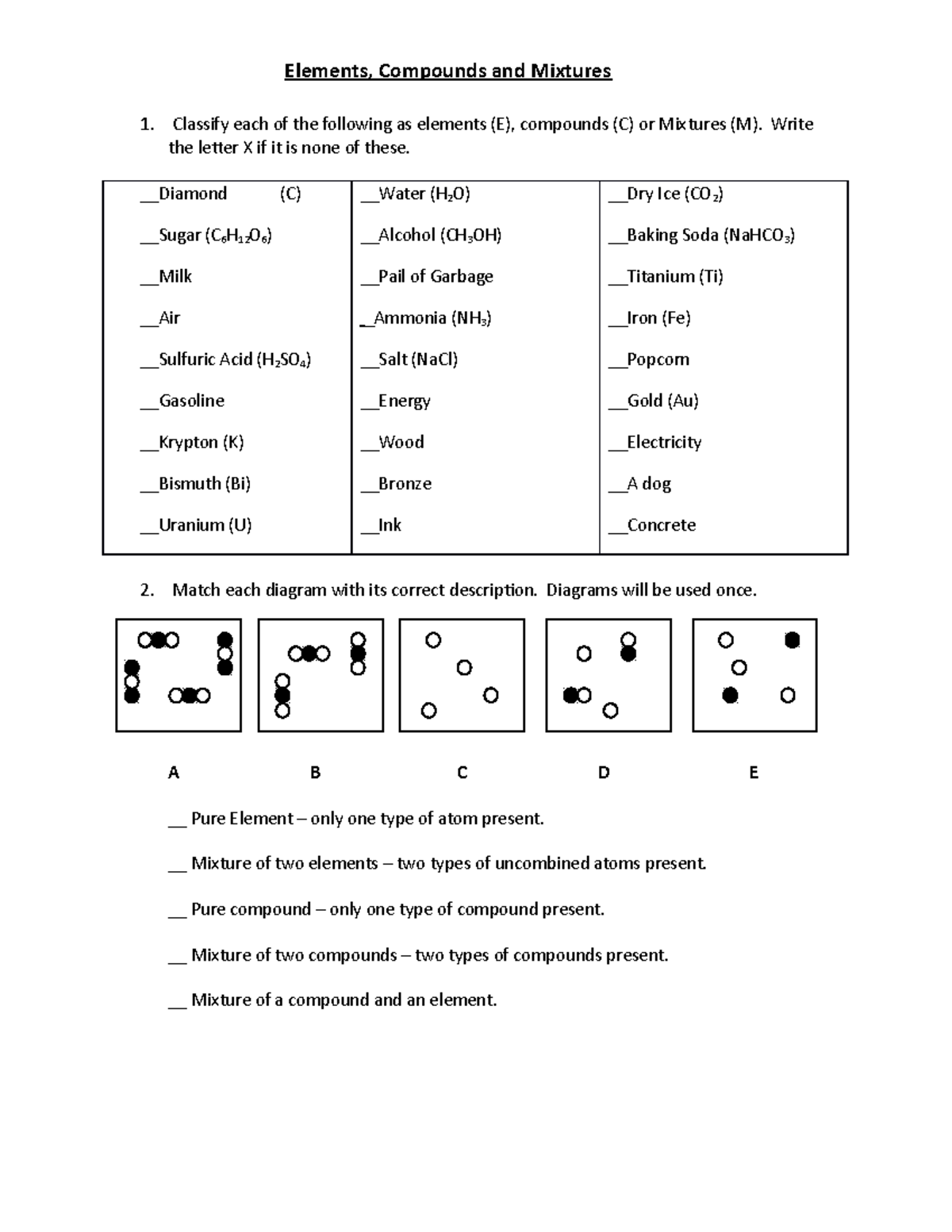 |
| Elements Compounds Mixtures Ws Elements Compounds And Mixtures Classify Each Of The Following As Studocu |
 |
| Creating Atomic Science Models Activity |
:max_bytes(150000):strip_icc()/illustration-of-properties-and-molecular-structure-of-water-consisting-of-two-hydrogen-atoms-covalently-bonded-to-single-oxygen-atom-85594579-583b267c5f9b58d5b1b9e84b-5bc4c2ea46e0fb0026923ba3.jpg) |
| Is Water A Compound Or An Element |
 |
| Answered 6 Determine Each Drawing Represents Bartleby |
 |
| Definition Of Compounds Elements Examples Types Classification With Videos |
Posting Komentar untuk "elements and compounds are two types of"